Which element,or combination of elements,could produce a semiconductor with the following band structure? (CB = conduction band,VB = valence band) 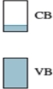
Definitions:
Q12: In which one of the following structures
Q41: For the reaction PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6485/.jpg"
Q51: What is the number of lone electron
Q54: Determine the freezing point of a solution
Q72: What types of elements undergo covalent bonding?<br>A)a
Q101: Carbon uses _ hybrid orbitals in ClCN.<br>A)sp<br>B)sp<sup>2</sup><br>C)sp<sup>3</sup><br>D)sp<sup>3</sup>d<br>E)sp<sup>3</sup>d<sup>2</sup>
Q106: In the valence bond treatment,overlap of an
Q111: Iodine trichloride,ICl<sub>3</sub>,will react with a chloride ion
Q124: "The volume of an ideal gas is
Q134: Which one of the following molecules is