Based on the phase diagram of a pure substance given below,which statement is true? 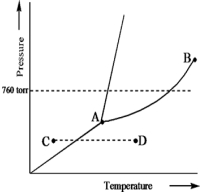
Definitions:
Organizational Behaviour
The study of how individuals and groups interact within organizations and how these interactions affect performance, motivation, job satisfaction, and leadership.
Prediction and Explanation
The process of forecasting future events or behaviors and providing reasons or causes for occurrences.
Managerial Task
Managerial tasks refer to the responsibilities and duties performed by managers, including planning, organizing, leading, and controlling to achieve organizational goals.
Field of Organizational Behaviour
A study area focused on understanding, explaining, and improving the attitudes and behaviors of individuals and groups in organizations.
Q43: A fixed quantity of gas,initially at 600ºC,undergoes
Q57: What is the vapor pressure above a
Q62: Octane is a component of fuel used
Q62: The observation that at equilibrium,the reaction quotient
Q64: Describe the chemicals used to make a
Q64: Which of the following contains ionic bonding?<br>A)CO<br>B)SrF<sub>2</sub><br>C)Al<br>D)OCl<sub>2</sub><br>E)HCl
Q83: A liquid boils when its<br>A)vapor pressure is
Q93: Nitric oxide and bromine were allowed to
Q115: The standard free energy of formation of
Q129: The equilibrium constant,K<sub>P</sub>,for the reaction H<sub>2</sub>(g)+ I<sub>2</sub>(g)