Essay
Consider the phase diagram shown below. 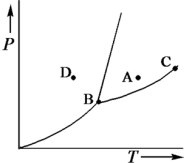 a.What phase(s)is/are present at point A?
a.What phase(s)is/are present at point A?
b.What phase(s)is/are present at point B?
c.Name point C and explain its significance.
d.Starting at D,if the pressure is lowered while the temperature remains constant,describe what will happen.
Evaluate the consequences of the Fed's purchase and sale of government securities on the banking system and the overall economy.
Understand the basic theories and models of social stratification and status attainment, including Blau and Duncan's and Wright's analyses.
Identify and explain the critical distinctions in class theory, particularly between property owners and non-owners, and how these distinctions impact power and opportunities.
Comprehend the functional and conflict theories of stratification and how they explain the distribution of rewards and roles within society.
Definitions:
Related Questions
Q2: An element with the general electron configuration
Q33: Which of these elements has the highest
Q44: _ is the term used for a
Q51: Which of these elements has the smallest
Q60: Which process defines how molecular compounds form
Q66: What is the name of the substance
Q67: In not more than two sentences,explain when
Q103: Sodium hydroxide is a common ingredient in
Q106: At -3°C,2.5 moles of a gas in
Q134: What is the percent CdSO<sub>4</sub> by mass