Based on the phase diagram of a pure substance given below,which statement is true? 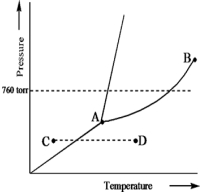
Definitions:
Price Rise
An increase in the price level of goods and services, often measured by inflation rates or observed in market price adjustments.
Comparative Advantage
The ability of a country to produce a good or service at a lower opportunity cost than its trading partners.
Fewer Resources
The state of having limited amounts of inputs required to produce goods and services, such as capital, labor, and materials.
Exchange Rates
The worth of a currency when converted into another, establishing the quantity of one currency that can be swapped for another.
Q27: Vulcanization<br>A)leads to cross-linking of poly-cis-isoprene chains in
Q49: Using the VSEPR model,what is the predicted
Q52: Explain the following,on the basis of osmosis
Q53: "The volume of an ideal gas is
Q56: In which of the following compounds will
Q81: Calculate the root-mean-square speed of methane,CH<sub>4</sub>(g),at 78°C.<br>A)23
Q82: The monomer for a polyester has the
Q85: The PCl<sub>5</sub> molecule has<br>A)nonpolar bonds,and is a
Q91: What name is given to the curved
Q106: Which of the responses includes all of