Consider the phase diagram shown below. 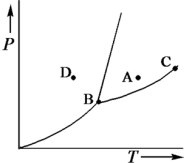 a.What phase(s)is/are present at point A?
a.What phase(s)is/are present at point A?
b.What phase(s)is/are present at point B?
c.Name point C and explain its significance.
d.Starting at D,if the pressure is lowered while the temperature remains constant,describe what will happen.
Definitions:
Important Priorities
Essential tasks or goals that require immediate attention and resources over less critical ones.
Allocation of Time
The process of planning and distributing one's available time among various activities and tasks.
Flexible Budget
A financial plan that adjusts or varies with changes in volume or activity levels.
Resource Allocation
The process of distributing available resources among various projects, processes, or units within an organization.
Q8: Ammonium iodide dissociates reversibly to ammonia and
Q20: The BrF<sub>5</sub> molecule has polar bonds and
Q22: According to the VSEPR model,a molecule with
Q30: What is the frequency of electromagnetic radiation
Q32: Solids are generally most stable in crystalline
Q48: Select the gas with the highest average
Q66: For any reaction,if ΔG° > 0,then K
Q80: At 850°C,the equilibrium constant,K<sub>P</sub>,for the reaction C(s)+
Q105: A flask with a volume of 3.16
Q108: A temperature increase favors an endothermic reaction.