The following is an Arrhenius plot of a first-order reaction.The rate constant is measured in units of s-1. 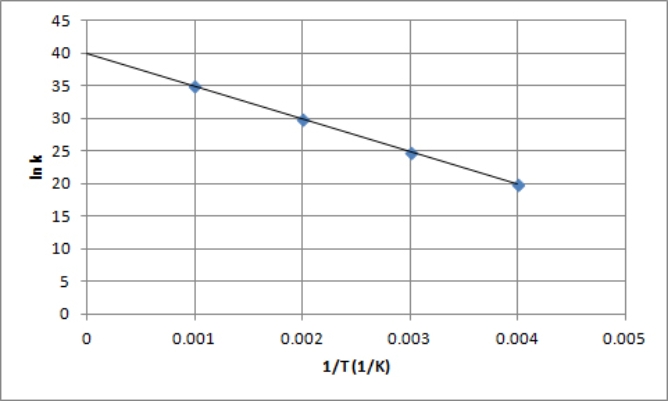 Based on this Arrhenius plot,what is the Arrhenius frequency factor (A) of the reaction? (R =8.314 J/K mol)
Based on this Arrhenius plot,what is the Arrhenius frequency factor (A) of the reaction? (R =8.314 J/K mol)
Definitions:
Price Leadership
Strategy where the leading firm in an industry sets the price of goods or services, which other firms in the market then follow or undercut.
Covert Collusion
An indirect or secret agreement among competitors to engage in anti-competitive behaviors, such as price-setting, that is not openly acknowledged or visible to regulatory authorities or the public.
Herfindahl-Hirschman Index
A measure of market concentration used to determine the level of competition within industries.
Kinked Demand Curve
A perceived market demand curve that suggests prices will rapidly decrease if a firm raises its prices above those of its competitors, but will not increase significantly if the firm lowers prices.
Q1: All of the following are a part
Q3: The commonly recognized development and learning domains
Q3: What can a teacher do to help
Q12: Each of the following statements is true
Q18: During which stage of Piaget's development should
Q21: The spectator ion is always included in
Q27: _,_,and _ are the three states of
Q29: When planning outdoor activities,teachers are responsible for
Q34: Potassium permanganate is a strong oxidizer that
Q55: The result of (3.8621 × 1.5630)- 5.98