Figure Q3-4 documents the quantal release of neurotransmitter. 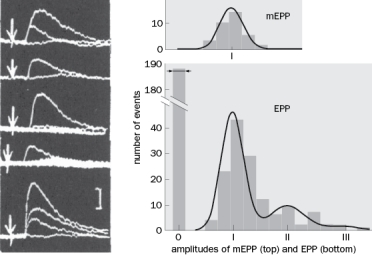 Figure Q3-4
Figure Q3-4
A. The investigators stimulated the nerve in low-Ca2+ and high-Mg2+ saline so that they had many failures with nerve stimulation. Why was the presence of failures so important?
B. In the top trace, there were three motor nerve stimulations that occurred at the arrow. Why is the flat trace flat and, based on what we know now, what is the reason that the other two traces are multiples of each other?
C. The investigators compared the evoked EPPs in low Ca2+/high Mg2+ saline to spontaneous EPPs (mEPP). What did they find (see figures on the right) and what did they conclude?
D. Based on the findings in this figure, what did they conclude about transmitter release in an evoked EPP in normal saline?
Definitions:
Deprotonated
Refers to the removal of a proton (H+) from a molecule, resulting in the formation of a negatively charged ion.
Resonance
A phenomenon in chemistry where a molecule or ion can be represented by two or more equivalent structures, indicating the delocalization of electrons.
Anion
A negatively charged ion, which has more electrons than protons.
CCC Bond Angle
The internal angle formed between three consecutively bonded carbon atoms in a molecule, often approximated to 109.5° in a tetrahedral arrangement.
Q9: Which of the following is not true
Q11: Game theory offers insight into:<br>A) pricing behavior
Q13: Compare the strategies bidders employ when participating
Q20: Which protein is the Ca<sup>2+</sup> sensor in
Q22: Why does the Na<sup>+</sup> conductance decrease after
Q30: Discuss the role of communication in a
Q32: In the pyloric circuit of the stomatogastric
Q38: Joint probability refers to:<br>A) the decision maker's
Q41: How can single, identifiable neurons be labeled?
Q41: Which is NOT an example of a