Which of the presented mechanisms would be the most energetically favorable and thus the most likely mechanism to actually occur for the following free radical chain reaction?
(bond dissociation energies - H-H = 104 kcal/mol; Cl-Cl = 58 kcal/mol; H-Cl = 103 kcal/mol)
H2 + Cl2 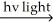 2 HCl
2 HCl
Definitions:
Physical Condition
The state of someone's or something's body or physical structure, often implying a level of health or fitness.
Stressful Situations
Scenarios that cause stress or anxiety, often requiring careful handling or adaptation.
Art Of Negotiation
The skillful practice of reaching mutually beneficial agreements or resolving disputes, involving techniques such as persuasion, strategic planning, and compromise.
Large Businesses
Corporations or enterprises that operate on a vast scale, often with extensive resources, employees, and market reach.
Q7: Show where chymotrypsin would cleave the peptide
Q20: A staffing issue and lack of patient
Q57: Which of the following cycloalkanes exhibits the
Q70: Provide a structure of the tripeptide SGD.
Q91: Explain which structural features of phospholipids make
Q92: Use the Hammond Postulate to explain why
Q114: Name two of the three predominant groups
Q119: A series of compounds, like the n-alkanes,
Q127: How many moles of carbon dioxide are
Q131: In a Newman projection, siting down the