Given an activation energy of 15 kcal/mol, use the Arrhenius equation to estimate how much faster the reaction will occur if the temperature is increased from 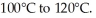
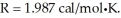
Definitions:
Projective Tests
Psychological assessments that use ambiguous stimuli to elicit responses that reflect the responder's feelings, desires, and conflicts.
Unconscious Motives
The underlying, often unaware reasons or forces that drive behaviors and decisions in individuals.
Diagnostic and Statistical Manual of Mental Disorders
A comprehensive classification of officially recognized psychiatric disorders, published by the American Psychiatric Association, serving as an authoritative guide for diagnosis.
Theoretical Assumptions
Foundational ideas that are accepted as true within a theoretical framework, guiding research and practice.
Q3: In the reaction of Cl<sub>2</sub> with ethane
Q3: Provide a reasonable synthesis of Gly-Val from
Q8: A patient tells the nurse that she
Q14: Which of the following statements is (are)
Q16: A clinic has instituted a program of
Q41: Draw the major organic product generated in
Q41: If the equilibrium constant (K<sub>eq</sub>) of a
Q86: Why are alkanes described as hydrophobic?
Q97: Predict the major monobromination product in the
Q121: Describe how soaps function as cleaning agents.