Which of the presented mechanisms would be the most energetically favorable and thus the most likely mechanism to actually occur for the following free radical chain reaction?
(bond dissociation energies - H-H = 104 kcal/mol; Cl-Cl = 58 kcal/mol; H-Cl = 103 kcal/mol)
H2 + Cl2 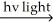 2 HCl
2 HCl
Definitions:
Statement Of Financial Position
A financial statement that provides a snapshot of a company's assets, liabilities, and shareholders' equity at a specific point in time, also known as a balance sheet.
IFRS 3
An International Financial Reporting Standard that deals with the accounting treatment for all business combinations.
Business Combinations
Transactions or events in which one entity gains control over one or more other businesses, often resulting in consolidations or acquisitions.
Parent-Company Method
An accounting approach where the parent company reports its investment in subsidiaries at cost, often used in separate financial statements.
Q20: A patient tells the nurse that she
Q32: In the molecule below, show how C3
Q35: Provide the reagents necessary to complete the
Q39: Which method gives you information about the
Q56: Provide the name of the compound shown.
Q70: Explain the stereochemical relationship, if any, among
Q81: Provide a detailed, stepwise mechanism for the
Q109: Why can methyl acrylate (H<sub>2</sub>C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6199/.jpg"
Q112: Provide an acceptable name for the alkane
Q134: Acid catalyzed hydration (H<sub>2</sub>SO<sub>4</sub>/water/△) of an unknown