Given an activation energy of 15 kcal/mol, use the Arrhenius equation to estimate how much faster the reaction will occur if the temperature is increased from 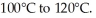
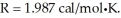
Definitions:
Self-Serving Bias
A common tendency for individuals to attribute positive outcomes to their own character but attribute negative outcomes to external factors.
Social Loafing
The phenomenon where individuals exert less effort to achieve a goal when they work in a group than when they work alone.
Deindividuation
A psychological state characterized by reduced self-awareness and decreased personal responsibility, often occurring in group situations that lead to uninhibited and impulsive behavior.
Social Contagion
The spread of behaviors, attitudes, and emotions through a group or society by social influence.
Q7: A patient is telling the nurse manager
Q28: How would you prepare CH<sub>3</sub>(CH<sub>2</sub>)<sub>14</sub>CH<sub>2</sub>NH<sub>2</sub> from palmitic
Q42: Provide the major organic product in the
Q43: Show how 3-methylthiopropanal may be converted to
Q68: What organic compounds result when tristearin is
Q88: Draw the major organic product generated in
Q100: Provide a synthesis of racemic valine from
Q113: Provide the major organic product of the
Q113: Which of the following describes the compound
Q132: Compounds that rotate the plane of polarized