The normalized wave function for a hydrogen atom in the 1s state is given by ψ(r) = 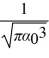 e-r/α0 where α0 is the Bohr radius, which is equal to 5.29 × 10-11 m. What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
e-r/α0 where α0 is the Bohr radius, which is equal to 5.29 × 10-11 m. What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
Definitions:
Payback
Payback is a capital budgeting metric that measures the time required for an investment to generate cash flows sufficient to recover the initial investment cost.
Mutually Exclusive
Situations, choices, or events that cannot occur or be chosen at the same time; selecting one option excludes the possibility of the other.
WACC
The Weighted Average Cost of Capital (WACC) represents the expected average return rate a company owes its security holders for financing its assets.
NPV
Net Present Value (NPV) is a financial metric used to evaluate the profitability of an investment, calculated by subtracting the present value of cash outflows from the present value of cash inflows over a period of time.
Q6: Two boxes are connected by a weightless
Q6: In the figure, a C-shaped conductor is
Q7: A star is moving towards the earth
Q11: The Corporations Act requires which of the
Q15: Which of the following are characteristics of
Q15: Within the principal/agent perspective of PAT, the
Q16: Two weights are connected by a massless
Q17: A rocket is moving at 1/4 the
Q17: A plate falls vertically to the floor
Q60: Which of the following statements about β<sup>+</sup>