Exhibit 6-10 Consider the Reaction of 2-Bromo-2-Methylpropane with Water,shown Below,to Answer the Answer
Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).
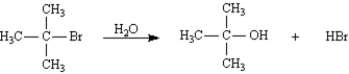
Diagram 1: The first step of this reaction is shown below.
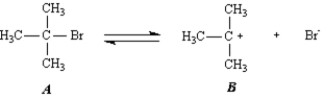 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.
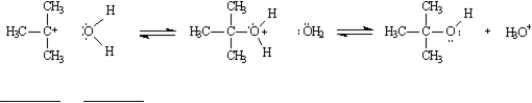
-Refer to Exhibit 6-10.Using the bond dissociations values in the table below,calculate the ΔH° for the reaction in Diagram 2.Show your calculations for full credit.
Definitions:
Q3: Draw: acetylene
Q5: Draw the resonance forms of 3,5-heptanedione anion.<br>
Q5: What is the skeletal formula for the
Q15: When 2-bromopropane reacts with ethoxide ion,two products
Q20: Refer to Exhibit 11-11.List all chirality centers
Q20: Name: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4944/.jpg" alt="Name: " class="answers-bank-image
Q22: The original question has been combined with
Q25: Which of the following is a characteristic
Q27: In general,5-alkyl substituents in 1,3-dioxane exhibit a
Q29: Which of the following is a common