The normalized wave function for a hydrogen atom in the 1s state is given by ψ(r) = 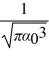 e-r/α0 where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
e-r/α0 where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
Definitions:
Paid-in Capital
The amount of capital "paid in" by investors during common or preferred stock issuances, including the par value of the shares themselves plus any amount paid in excess.
Stockholders' Equity
The residual interest in the assets of a corporation after deducting liabilities, representing owners' claims on the company's resources.
Treasury Stock
Shares initially released to the public and then bought back by the corporation that issued them, diminishing the overall supply of shares on the market.
Paid-in Capital
The amount of money that a company has received from shareholders in exchange for shares of stock, above the par value of the shares.
Q13: In a diffraction grating experiment,light of 600
Q14: A constructor of the base class is
Q19: A stack is a specialized type of
Q26: Near the earth the intensity of radiation
Q27: Monochromatic laser light of frequency 5.20 ×
Q32: The arrow operator ->)specifies<br>A)a member of a
Q39: A struct variable is declared differently from
Q52: A 200-loop coil of cross sectional area
Q57: A ray of light traveling in air
Q62: At a certain instant the current flowing