A 610-g quantity of an ideal gas undergoes a reversible isothermal compression at a temperature of 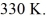 The compression reduces the volume of the gas from
The compression reduces the volume of the gas from 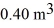 initially,to
initially,to 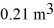 finally.The molecular mass of the gas is
finally.The molecular mass of the gas is 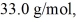 and the ideal gas constant is R = 8.314 J/(mol ∙ K) .The entropy change for the gas is closest to
and the ideal gas constant is R = 8.314 J/(mol ∙ K) .The entropy change for the gas is closest to
Definitions:
Uncollectible Account Receivable
An accounting term for receivables from sales that are considered not collectible, resulting in a bad debt expense.
Uncollectible Accounts
Accounts receivable that a company does not expect to collect and thus writes off as a loss.
Direct Write-off Method
An accounting practice where uncollectable debts are written off against income at the time they are deemed non-collectable.
Allowance Method
An accounting technique that estimates uncollectible accounts receivable to record bad debts expense, reflecting more accurate accounts.
Q5: Calculate the light intensity 1.51 m from
Q12: Building codes usually limit the current carried
Q22: When a potential difference of 10 V
Q40: For the wave shown in the figure,the
Q47: A satellite of mass m has an
Q47: What is the energy density in the
Q49: The gas in a perfectly insulated system
Q54: If you double the pressure on the
Q56: A loop of radius r = 3.0
Q64: A cylinder contains 1.2 moles of ideal