A substance has a melting point of 20°C and a heat of fusion of 3.9 × 104 J/kg.The boiling point is 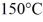 and the heat of vaporization is 7.8 × 104 J/kg at a pressure of 1.0 atm.The specific heats for the solid,liquid,and gaseous phases are 600 J/(kg∙K) ,1000 J/(kg∙K) ,and 400 J/(kg∙K) ,respectively.The quantity of heat required to raise the temperature of
and the heat of vaporization is 7.8 × 104 J/kg at a pressure of 1.0 atm.The specific heats for the solid,liquid,and gaseous phases are 600 J/(kg∙K) ,1000 J/(kg∙K) ,and 400 J/(kg∙K) ,respectively.The quantity of heat required to raise the temperature of 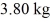 of the substance from
of the substance from 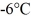 to
to 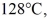 at a pressure of 1.0 atm,is closest to
at a pressure of 1.0 atm,is closest to
Definitions:
Formatting Instructions
Guidelines or commands that specify how data should be presented or formatted, often used in programming to improve the readability or appearance of output.
System.out.println
A Java statement used to print a line of text to the console or output stream.
Arguments
Values or variables passed to a function or method when it is called, used within the function to perform operations or calculations.
Java Package
A mechanism in Java used to group related classes and interfaces together, making the code easier to manage and modular.
Q2: Is it possible to transfer heat from
Q13: A sample of an ideal gas is
Q13: The current supplied by a battery as
Q21: A galvanometer coil having a resistance of
Q22: A Carnot cycle engine operates between a
Q38: The cross section of a long coaxial
Q41: Two long parallel wires carry currents of
Q41: A 2.00-kg object is attached to an
Q45: A mass M is attached to an
Q90: A substance has a melting point of