An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram.The initial pressure is 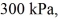 the initial volume is
the initial volume is 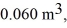 and the initial temperature is
and the initial temperature is 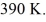 The ideal gas constant is R = 8.314 J/mol ∙ K.The final pressure is
The ideal gas constant is R = 8.314 J/mol ∙ K.The final pressure is 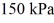 and the final temperature is
and the final temperature is 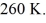 The work done by the gas is closest to
The work done by the gas is closest to
Definitions:
Acquisition Cost
The total cost associated with acquiring an asset or investment, including the purchase price and all related expenses.
Due Diligence Fees
Costs associated with the research and analysis conducted before entering into an agreement or transaction.
Fair Value
The financial gain on unloading an asset or the financial duty to convey a liability in a coordinated market activity at the established valuation date.
Costs of Issuing
Expenses associated with the issuance of stocks or bonds, including underwriting, legal, and registration fees.
Q1: As shown in the figure,a square insulating
Q4: A wire along the z-axis carries a
Q11: A small-sized 155-kg mass is located 1.50
Q21: A solid nonconducting sphere of radius R
Q24: A cylindrical wire of radius 2.0 mm
Q31: It is a well-known fact that water
Q36: A -3.0-μC point charge and a -9.0-μC
Q38: The figure (not to scale)shows a pV
Q63: How much work is done by 3.00
Q108: A force <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4470/.jpg" alt="A force