The figure shows the pV diagram for a certain thermodynamic process.In this process,
1500 J of heat flows into a system,and at the same time the system expands against a constant external pressure of 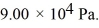 If the volume of the system increases from
If the volume of the system increases from 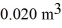 to
to 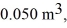 calculate the change in internal (thermal)energy of the system.If the internal (thermal)energy change is nonzero,be sure to indicate whether this energy change is positive or negative.
calculate the change in internal (thermal)energy of the system.If the internal (thermal)energy change is nonzero,be sure to indicate whether this energy change is positive or negative. 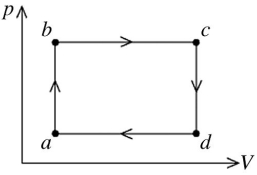
Definitions:
Growth
Growth refers to the process of increasing in size, strength, or complexity, often seen in biological, personal, or economic contexts.
Duration
The duration in which something persists or remains ongoing.
Motivation
The process that initiates, guides, and maintains goal-oriented behaviors, driven by the desire to fulfill a need or achieve an objective.
Constant Flow
A steady, unchanging rate of movement or supply of a fluid, energy, or another quantifiable phenomenon.
Q4: A long,thin rod parallel to the y-axis
Q5: Consider the group of three+2.4 nC point
Q7: The graph in the figure shows a
Q11: The x component of the velocity of
Q14: A 1.0-kg block and a 2.0-kg block
Q24: An ideal Carnot engine operates between reservoirs
Q24: A pipe is 0.90 m long and
Q27: A box of mass m is pressed
Q29: A frictionless pendulum clock on the surface
Q54: An ideal gas increases in temperature from