An unknown compound X has the empirical formula C3H6O and a molecular ion in its mass spectrum at 116.Compound X shows no IR absorption at 3200-3600 cm-1 but shows a peak at 1700 cm-1.The 1H NMR spectral data of X is shown below.What is the structure of compound X? 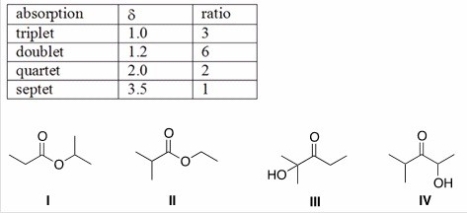
Definitions:
Slippery Slope Fallacy
A logical fallacy in which a relatively small first step leads to a chain of related events culminating in some significant effect, much like sliding down a slippery slope.
Gambler's Fallacy
A logical fallacy in which one assumes that future probabilities are altered by past events, often seen in gambling when assuming a certain outcome is "due".
Sunk Cost Fallacy
The misconception of valuing a project or investment based on the amount of resources already invested, rather than the prospective future returns.
Argumentum Ad Hominem
A logical fallacy that occurs when an argument is rebutted by attacking the character, motive, or other attribute of the person making the argument, rather than addressing the substance of the argument itself.
Q6: Which of the following statements is (are)true
Q14: In ancient Egyptian art,which of the following
Q15: The works by Johannes Vermeer demonstrate the
Q17: Which of the following π bonds is
Q21: In Buddha Shakyamuni,the ushnisha symbolizes the Buddha's
Q21: In the Sphinx of Taharqo,the facial features
Q23: The first Gothic building was _.<br>A)Reims Cathedral<br>B)Chartres
Q24: What is the setting for Robert Campin's
Q27: An unknown compound X has the molecular
Q48: Predict the major organic product(s)of the following