What is the percent yield for the reaction PCl3(g) + Cl2(g) → PCl5(g)
If 119.3 g of PCl5 (M = 208.2 g/mol) are formed when 61.3 g of Cl2 ( 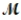 = 70.91 g/mol) react with excess PCl3?
= 70.91 g/mol) react with excess PCl3?
Definitions:
Group Is Aggressive
A situation where a collective behaves in a forceful or assertive manner, often to achieve a common goal.
Big-C Creativity
What occurs when extraordinary things are done by exceptional people.
Unique Ways
Innovative or distinctive methods or approaches to doing something.
Availability Bias
A cognitive bias that influences people to overestimate the importance of information available to them, impacting decision-making.
Q3: Select the precipitate that forms when aqueous
Q7: The more C-O and O-H bonds there
Q9: Cesium-134 is a β emitter with a
Q11: Magnesium reacts with iron(III) chloride to form
Q18: A system expands from a volume of
Q23: One mole of methane (CH<sub>4</sub>) contains a
Q49: Which of the following species could exist
Q66: A system that does no work but
Q80: What is the name of PCl<sub>3</sub>?<br>A) phosphorus
Q89: A particular reaction may be both a