Calculate the equilibrium constant at 25°C for the reaction of methane with water to form carbon dioxide and hydrogen. The data refer to 25°C. CH4(g) + 2H2O(g)  CO2(g) + 4H2(g)
CO2(g) + 4H2(g) 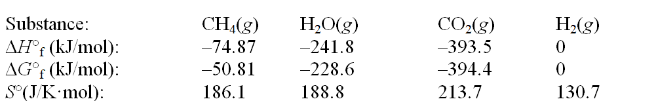
Definitions:
Accounts Payable
Liabilities of a company or organization representing money owed to suppliers or creditors for goods and services received.
Dividends Paid
The portion of a company's earnings that is distributed to shareholders, typically in the form of cash payments or additional stock.
Net New Investments
This refers to the total amount of money invested in new asset purchases minus any sales of assets over a specific period, reflecting a company's growth investment activities.
Cash Flow From Investing
Cash flow from investing activities is a section of a company's cash flow statement that shows the inflow and outflow of cash resulting from investment activities, such as purchases or sales of physical assets or investments in securities.
Q11: Consider the equilibrium reaction: H<sub>2</sub>(g) + Br<sub>2</sub>(g)
Q15: The process used to produce silicon with
Q16: Changing the amount of a solid reactant
Q28: What is the pH of a buffer
Q31: Ammonia, an important source of fixed nitrogen
Q33: Tetraphosphorus hexaoxide ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg" alt="Tetraphosphorus hexaoxide
Q44: The nuclide Pb-210 undergoes three successive decays
Q49: For a chemical reaction to be non-spontaneous
Q76: Nitric oxide is formed in automobile exhaust
Q87: What is the mass-action expression, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg"