The reaction of methane with water to form carbon dioxide and hydrogen is nonspontaneous at 298 K. At what temperature will this system make the transition from nonspontaneous to spontaneous? The data refer to 298 K. CH4(g) + 2H2O(g)  CO2(g) + 4H2(g)
CO2(g) + 4H2(g) 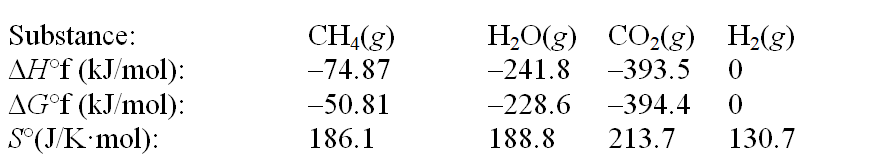
Definitions:
Market Price
The market rate for buying or selling an asset or service in a public trading environment.
Producer Surplus
The gap between the minimum amount sellers are prepared to accept for a product or service and the actual payment they get.
Equilibrium
The point at which the quantity of a product or service demanded by consumers equals the quantity supplied by producers, leading to a stable market condition.
Market Equilibrium
The condition in which a market's supply and demand balance each other, and as a result, prices become stable.
Q6: Aqueous potassium iodate (KIO<sub>3</sub>) and potassium iodide
Q8: The temperature at which the following process
Q11: Which of the following is considered a
Q22: The kinetics of the decomposition of dinitrogen
Q35: During a titration, the following data were
Q37: A certain transition element has the stable
Q45: The rate law for the reaction 3A
Q52: Carbon-14 is a radioactive isotope which decays
Q58: If the equilibrium constant K<sub>c</sub> is greater
Q72: The inner transition series of elements arise