Liquid ammonia (boiling point = -33.4°C) can be used as a refrigerant and heat transfer fluid. How much energy is needed to heat 25.0 g of NH3(l) from -65.0°C to -12.0°C?
Specific heat capacity, NH3(l) : 4.7 J/(g·K)
Specific heat capacity, NH3(g) : 2.2 J/(g·K)
Heat of vaporization: 23.5 kJ/mol
Molar mass, 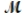 : 17.0 g/mol
: 17.0 g/mol
Definitions:
Piaget's Theory
A developmental theory by Jean Piaget that outlines how children's cognitive abilities progress through four stages from infancy to adolescence.
Abstract Reasoning
The ability to analyze information, detect patterns and relationships, and solve problems on a complex, abstract level.
Moral Development
The growth of an individual's understanding of the principles and values that guide responsible behavior.
Lawrence Kohlberg
A psychologist best known for his theory of the stages of moral development, which describes how individuals evolve in their understanding of morality.
Q10: Public relations activities can be used to
Q22: A salesperson would be most likely to
Q36: In formaldehyde, CH<sub>2</sub>O, both the formal charge
Q44: A characteristic reaction of alkanes is addition.
Q47: To encourage sales, a candy maker has
Q51: What is the mass-action expression, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg"
Q56: Which of the following is an input
Q62: Only molecules that do not have dipole
Q86: In which one of the following molecules
Q91: Which of the following formulas does not