What quantity,in moles,of hydrogen is consumed in the reaction below when 307.6 kJ of energy is evolved from the combustion of a mixture of hydrogen gas and oxygen gas? H2(g) + 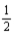 O2(g) → H2O(l) ; ΔrH° = -285.8 kJ/mol-rxn
O2(g) → H2O(l) ; ΔrH° = -285.8 kJ/mol-rxn
Definitions:
Beta-Endorphins
Natural peptides produced by the body that act as neurotransmitters to reduce pain and promote feelings of well-being.
Aerobic Exercise
Physical activity that improves cardiovascular health by increasing the heart rate and oxygen intake for a prolonged period.
Anxiety
A feeling of worry, nervousness, or unease about something with an uncertain outcome.
Body Mass Index (BMI)
A measure of body fat based on height and weight that applies to adult men and women.
Q7: When <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="When undergoes
Q28: Which of the following is the by-product
Q33: Give two examples of the allotropes of
Q39: If Δ<sub>r</sub>G° > 0 for a reaction
Q56: A current of 12.0 A is passed
Q60: A first-order reaction is 40.0% complete at
Q61: Identify the orbital shown below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg"
Q135: Complete the following fission reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg"
Q177: Choose the pair in which the components
Q186: Write the name for Kr._