Calculate 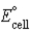 for the electrochemical cell Pb(s) |PbCl2(s) | Cl-(aq,1.0 M) || Fe3+(aq,1.0 M) ,Fe2+(aq,1.0 M) | Pt(s) . The standard reduction potentials are as follows:
for the electrochemical cell Pb(s) |PbCl2(s) | Cl-(aq,1.0 M) || Fe3+(aq,1.0 M) ,Fe2+(aq,1.0 M) | Pt(s) . The standard reduction potentials are as follows:
Pb2+(aq) + 2 e- → Pb(s)
E° = -0.126 V
PbCl2(s) + 2 e- → Pb(s) + 2 Cl-(aq)
E° = -0.267 V
Fe3+(aq) + e- → Fe2+(aq)
E° = +0.771 V
Fe2+(aq) + e- → Fe(s)
E° = -0.44 V
Definitions:
Gamification
The application of game-design elements and principles in non-game contexts to engage and motivate people to achieve their goals.
Mastery
A high level of skill or knowledge in a particular subject or activity.
Frequency Marketing
A marketing strategy designed to reward customer loyalty by providing benefits or rewards based on the frequency of purchase or engagement.
Behavioural Learning Theory
A theory that explains learning in terms of observable behaviors, emphasizing the effects of external stimuli on one's actions.
Q6: Which of the following methods is used
Q12: What is the molar solubility of solid
Q20: Which of the following complexes has linkage
Q23: Radioactive isotopes decay by _-order kinetics.
Q30: Which of the following cannot be used
Q31: Henry's Law constant is 0.0013 mol/kg⋅bar and
Q40: What is the H<sub>3</sub>O<sup>+</sup> concentration in 0.0055
Q49: Calculate <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="Calculate for
Q65: A 50.00-mL solution of 0.0426 M trimethylamine
Q72: Which of the following is the correct