If ΔrG° for the following reaction is -22.2 kJ/mol-rxn,calculate 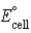 for the following reaction: Cu2+(aq) + 2 Ag(s) + 2 Cl-(aq) → Cu(s) + 2 AgCl(s)
for the following reaction: Cu2+(aq) + 2 Ag(s) + 2 Cl-(aq) → Cu(s) + 2 AgCl(s)
Definitions:
Producer Surplus
The difference between the amount producers are willing to sell a good for and the amount they actually receive.
Price Discrimination
A pricing strategy where identical or substantially similar goods or services are sold at different prices by the same provider in different markets.
Seniors
Older adults, typically those aged 65 and above, who may qualify for certain benefits and considerations due to their age.
Students
Individuals engaged in the process of learning, typically within an educational institution such as a school or university.
Q8: _ are colloidal dispersions of one liquid
Q11: How many neutrons are present in a
Q25: The oxygen that bridges two sugars in
Q26: A molecule that can behave as either
Q27: Assuming ideal behavior,which of the following aqueous
Q35: The products of the reaction of strontium
Q50: A solution containing 10.mmol of CO<sub>3</sub><sup>2</sup><sup>−</sup> and
Q71: What is the name of the given
Q88: What is the pH of the solution
Q94: Given that K<sub>a</sub> for the weak acid