Given that Ka for the weak acid HA is 3.49 × 10-8,calculate K for the reaction of HA with OH-. HA(aq) + OH−(aq) D A−(aq) + H2O( 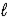 )
)
Definitions:
Social Loafing
The phenomenon where individuals exert less effort to achieve a goal when they work in a group than when they work alone.
Ingroup Bias
The tendency to favor members of one's own group over those from external groups, often leading to prejudice and discrimination.
Individual Performance
Individual performance is the measure of how well a person accomplishes their tasks or goals in a specific context, such as work or sports.
Group Polarization
A tendency for group discussion to shift group members toward an extreme position.
Q5: What is the oxidation state of nitrogen
Q12: Which of the following statements about entropy
Q25: If a cube of ice at 0
Q26: An unknown white solid was found to
Q33: For the overall reaction A + 2B
Q42: What is the mass percent of an
Q64: If an egg's shell is carefully dissolved
Q79: Ammonium perchlorate can decompose violently according to
Q83: What is the concentration of silver(I)ion in
Q86: A buffer contains 0.50 mol NH<sub>4</sub><sup>+</sup> and