Batteries used in watches contain mercury(II) oxide.As the current flows,mercury(II) oxide is reduced to mercury according to the following reaction: HgO(s) + H2O( 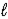 ) + 2 e- → Hg(
) + 2 e- → Hg( 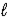 ) + 2 OH-(aq)
) + 2 OH-(aq)
If 2.3 × 10-5 amperes flows continuously for 1200 days,calculate the mass of mercury,Hg( 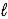 ) ,produced.
) ,produced.
Definitions:
Dominant Genes
Genes that overpower their recessive counterparts and determine the phenotype of an organism.
Heterozygous Pairs
In genetics, refers to a pair of genes where one is dominant and the other is recessive; they're different alleles for a specific trait.
Gonads
The reproductive organs in animals and humans that produce gametes; testes in males and ovaries in females.
Adrenal Glands
Small glands located on top of each kidney, responsible for producing hormones that regulate metabolism, immune system, blood pressure, and stress responses.
Q27: Given the equilibrium constants for the equilibria,
Q33: When a real gas is compressed from
Q35: What is the S0 for ozone if
Q36: A 50.00-mL solution of 0.0350 M ethylamine
Q39: What is the mass of H<sub>2</sub>SO<sub>4</sub> in
Q39: Aluminum(III)ion (Al<sup>3+</sup>)is reduced to solid aluminum at
Q41: What is the balanced chemical equation for
Q56: ΔG° = 0 for a reaction indicates
Q59: What is the pH of a buffer
Q61: A sample of solid NH<sub>4</sub>NO<sub>3</sub> was placed