Calculate the standard cell potential ( 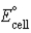 ) for the reaction 2 Ag(s) + Co2+(aq) → 2 Ag+(aq) + Co(s) . The standard reduction potentials are as follows:
) for the reaction 2 Ag(s) + Co2+(aq) → 2 Ag+(aq) + Co(s) . The standard reduction potentials are as follows:
Ag+(aq) + e−→ Ag(s)
E° = 0.8 V
Co2+(aq) +2 e−→ Co(s)
E° = -0.277 V
Definitions:
Job Analysis
A systematic process for gathering, documenting, and analyzing information about the content, requirements, and context of a job.
Sales Position
A role within a company focused on selling products or services, typically involving activities like prospecting for new clients, presenting products, and closing sales.
Tasks of the Job
The set of responsibilities, duties, and activities that are expected to be performed by an individual holding a specific position.
Account Management Policy Grid
A strategic framework used to classify and manage a company's customers or accounts based on their value and the complexity of the account to optimize service and resources.
Q4: The standard reduction potentials for a reaction
Q19: The rate constant for a particular reaction
Q25: The concentration of calcium carbonate in a
Q27: What is the pH of a buffer
Q27: Which of the following reactions will require
Q38: The carbon-carbon bonding of cyclopropane is shown
Q46: What is the hydronium-ion concentration in a
Q76: Which of the following elements is an
Q90: What is the pH of a 0.42
Q172: Which pair has approximately the same mass?<br>A)