Calculate the equilibrium constant for the following reaction at 25 °C, 2 IO3-(aq) + 5 Hg( 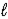 ) + 12 H+(aq) → I2(s) + 5 Hg2+(aq) + 6 H2O(
) + 12 H+(aq) → I2(s) + 5 Hg2+(aq) + 6 H2O( 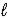 )
)
The standard reduction potentials are as follows:
IO3-(aq) + 6 H+(aq) + 5 e- → I2(s) + 3 H2O( 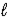 )
)
E° = +1.20 V
Hg2+(aq) + 2 e- → Hg( 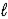 )
)
E° = +0.86 V
Definitions:
Self-Report Measure
A type of psychological assessment where individuals provide subjective reports about their own thoughts, feelings, or behaviors.
Personality Assessment
The way we measure and capture personality, using a variety of methods.
Cronbach's Alpha
A measure used in statistics to assess the reliability, or internal consistency, of a set of scale or test items.
Internal Reliability
A measure of consistency within a psychological test, indicating how closely related the items in the test are as a group.
Q14: Amino acids polymerize in condensation reactions that
Q17: Which of the following is correct for
Q19: Which of the following functional groups are
Q51: At 25 °C,the decomposition of dinitrogen tetraoxide
Q55: For which of the following reactions is
Q61: A(n)_ reaction is one where an element
Q61: CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>COOH is _.<br>A) isobutyl alcohol<br>B) butyl alcohol<br>C)
Q63: What mass of sodium hydroxide must be
Q73: The solubility of 1-pentanol in water is
Q88: What is the pH of the solution