Calculate 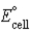 for the electrochemical cell Pb(s) |PbCl2(s) | Cl-(aq,1.0 M) || Fe3+(aq,1.0 M) ,Fe2+(aq,1.0 M) | Pt(s) . The standard reduction potentials are as follows:
for the electrochemical cell Pb(s) |PbCl2(s) | Cl-(aq,1.0 M) || Fe3+(aq,1.0 M) ,Fe2+(aq,1.0 M) | Pt(s) . The standard reduction potentials are as follows:
Pb2+(aq) + 2 e- → Pb(s)
E° = -0.126 V
PbCl2(s) + 2 e- → Pb(s) + 2 Cl-(aq)
E° = -0.267 V
Fe3+(aq) + e- → Fe2+(aq)
E° = +0.771 V
Fe2+(aq) + e- → Fe(s)
E° = -0.44 V
Definitions:
Ordinary annuity
Regular payments of the same amount made at set intervals, during which interest is calculated and added at the end of each period.
Conditional sale contract
An agreement where the sale is conditional upon certain terms, often used in property and vehicle sales where possession is granted but legal ownership is retained by the seller until full payment is received.
Compounded monthly
The process of adding interest to the principal sum of a loan or deposit, where the interest is added monthly.
Compounded monthly
The process of calculating interest on an investment or loan each month, with each month's interest added to the principal.
Q3: Which of the following expressions does not
Q7: What is the formula for dicyanobis(ethylenediamine)zirconium(IV)nitrate? (en
Q14: Which of the following is true of
Q19: Which of the following is the cell
Q29: For the reaction A → B +
Q38: Nitrogen and oxygen gases may react to
Q80: Which of the following indicators is most
Q81: Hydrofluoric acid has a pK<sub>a</sub> value of
Q153: Which of the following is a metalloid?<br>A)
Q162: The number of protons in the nucleus