Nitrogen and oxygen gases may react to form nitrogen monoxide.At 1500 °C,Kc equals 1.0 × 10−5. N2(g) + O2(g) 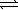 2 NO(g)
2 NO(g)
If 0.030 mol N2 and 0.030 mol O2 are sealed in a 1.0 L flask at 1500 °C,what is the concentration of NO(g) when equilibrium is established?
Definitions:
Variable Manufacturing Overhead
Costs that vary in total in direct proportion to changes in the volume of production, such as indirect materials and utility costs directly tied to production.
Raw Materials
The basic materials from which a product is made, prior to any processing or manufacturing.
Price Variance
The difference between the actual price paid for something and its standard or expected price.
Labor Efficiency Variance
The difference between the actual hours worked and the standard hours allowed for the work done, multiplied by the standard labor rate.
Q8: Hydrazine,N<sub>2</sub>H<sub>4</sub>,is produced by the Raschig process−the oxidation
Q13: Cesium crystallizes in the body-centered cubic system.If
Q22: The symbol Q is called the _.
Q34: Which of the following is the correct
Q38: For a chemical reaction,if Δ<sub>r</sub>G° = 0,then
Q43: What is the osmotic pressure of an
Q51: How many mechanistic steps are depicted by
Q53: Refer to Diagram 9-1.According to molecular orbital
Q66: What is the pH at the equivalence
Q80: When a Lewis acid and a Lewis