If ΔrG° for the following reaction is -22.2 kJ/mol-rxn,calculate 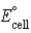 for the following reaction: Cu2+(aq) + 2 Ag(s) + 2 Cl-(aq) → Cu(s) + 2 AgCl(s)
for the following reaction: Cu2+(aq) + 2 Ag(s) + 2 Cl-(aq) → Cu(s) + 2 AgCl(s)
Definitions:
Policies Benefit Society
Policies that benefit society are designed to improve the well-being of the general public and the social structure as a whole.
Award For Corporate Excellence
A recognition given to companies that exemplify outstanding performance in areas such as social responsibility, innovation, and ethical behavior.
Social Responsibility Overseas
Refers to the duties and ethical considerations companies undertake to benefit society and the environment outside their home country.
Integrated Marketing Communication
A strategic approach that combines various traditional and digital marketing methods, such as advertising, public relations, and social media, to create a unified and consistent message across all channels.
Q1: How many moles of HCl must be
Q11: Which of the following ionic compounds forms
Q11: The K<sub>sp</sub> of BaSO<sub>4</sub> is 1.1 ×
Q20: The chemical formula Al<sub>2</sub>O<sub>3</sub> indicates:<br>A) two atoms
Q30: Nitrogen trifluoride decomposes at to form nitrogen
Q46: Sulfuryl chloride decomposes to sulfur dioxide and
Q54: What is the equilibrium constant for reaction
Q58: Which of the following species have geometric
Q85: What is the concentration of Cd<sup>2+</sup>(aq)in a
Q107: Which of the following isotopes is used