The value of E°cell is 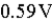 for the following reaction: Cl2(g) + 2 Fe2+(aq) → 2 Fe3+(aq) + 2 Cl-(aq)
for the following reaction: Cl2(g) + 2 Fe2+(aq) → 2 Fe3+(aq) + 2 Cl-(aq)
Calculate the value of E°cell for the reaction below.
Cl-(g) + Fe3+(aq) → Fe2+(aq) + ½ Cl2(g)
Definitions:
Q7: The reaction quotient,Q,for a system is <img
Q16: Below is a list of complex ions
Q16: Which of the following layers of the
Q20: What mass of Na<sub>2</sub>SO<sub>4</sub> must be dissolved
Q30: Bauxite ore is the primary source material
Q45: When a secondary battery provides electrical energy,it
Q55: How many milliliters of 11.7 M H<sub>2</sub>SO<sub>4</sub>
Q61: CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>COOH is _.<br>A) isobutyl alcohol<br>B) butyl alcohol<br>C)
Q71: The active ingredients in "strike anywhere" matches
Q86: Calculate the cell potential at 25 °C