The reaction quotient,Q,for a system is 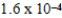 .If the equilibrium constant for the system at some temperature is
.If the equilibrium constant for the system at some temperature is 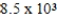 ,what will happen as the reaction mixture returns to equilibrium?
,what will happen as the reaction mixture returns to equilibrium?
Definitions:
Fixed Costs
Costs that do not change with the level of production or business activity, such as rent, salaries, and insurance premiums.
Full-Service Contract
An agreement that provides a complete range of services related to a specific product, property, or activity, including maintenance, repairs, and often replacements.
Profit
The financial gain achieved when the revenue from business activities exceeds the expenses, costs, and taxes involved in maintaining the operation.
Operating Loss
A financial metric representing the negative balance resulting from a company's operating expenses exceeding its revenues.
Q1: Which of the following pairs of species
Q7: For a certain reaction of the general
Q14: Which of the following reactions does not
Q34: Which of the following is the bond
Q43: An impure sample of sodium carbonate,Na<sub>2</sub>CO<sub>3</sub>,is titrated
Q44: What is the equilibrium concentration of H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>
Q66: London dispersion forces are the only significant
Q68: The standard enthalpy of formation of ammonia
Q78: All of the following compounds are acids
Q82: Arrange the following acids in order of