Calculate ΔrG° at 25.0 °C for the reaction below. 2 Na(s) + 2 H2O( 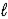 ) → 2 NaOH(aq) + H2(g)
) → 2 NaOH(aq) + H2(g)
Given: ΔrH° = −366.6 kJ/mol-rxn and ΔrS° = −154.2 J/K⋅mol-rxn.
Definitions:
Training Program
A structured sequence of education and activities designed to enhance the skills, knowledge, and competencies of individuals for a specific role or job.
Goal Setting
The process of identifying specific, measurable, achievable, relevant, and time-bound objectives.
Performance Appraisal
A systematic evaluation of an employee's job performance and productivity in relation to certain pre-established criteria and organizational objectives.
Desired Outcomes
The specific results or goals that an individual or organization aims to achieve through its actions or interventions.
Q3: What is the product of the reduction
Q5: What is the equilibrium constant for the
Q12: Point D on the phase diagram is
Q13: How many unique tripeptides can be made
Q26: A molecule that can behave as either
Q59: The following equation is known as _
Q61: A(n)_ reaction is one where an element
Q70: The hydroxide ion concentration of a saturated
Q71: Use the following standard reduction potentials to
Q72: Which of the following statements about soaps