Use the following standard reduction potentials to determine which species is the best oxidizing agent. O2(g) + 4 H+(aq) + 4 e- → 2 H2O( 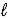 )
)
E° = +1.229 V
Hg22+(aq) + 2 e- → 2 Hg( 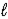 )
)
E° = +0.789 V
I2(s) + 2 e- → 2 I-(aq)
E° = +0.535 V
Definitions:
Brain Structure Abnormalities
Deviations or anomalies in the anatomy of the brain that can influence behavior and cognitive function.
Biopsychosocial Approach
A comprehensive outlook that merges biological, psychological, and socio-cultural aspects to analyze and grasp the concepts of health and illness.
Genetic Predispositions
The increased likelihood of developing a particular disease based on a person's genetic makeup.
Psychological Dynamics
The underlying causes and processes that contribute to psychological phenomena or behavior.
Q1: Which of the following pairs of species
Q5: Which of the following statements is true
Q6: The oxidation state per each nitrogen,given in
Q21: Biodiesel is a mixture of:<br>A) fats and
Q22: Calculate the equilibrium constant for the following
Q27: Which of the following statements is INCORRECT?<br>A)
Q33: Which of the following is the correct
Q41: In an octahedral complex,electrons in <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg"
Q51: A buffer is composed of 0.400 mol
Q57: Write the symbol for carbon._