Calculate the molar concentration of uncomplexed Zn2+(aq) in a solution that contains 0.17 M Zn(NH3) 42+ and 0.3775 M NH3 at equilibrium.Kf for Zn(NH3) 42+ is 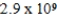 .
.
Definitions:
Gasoline
A volatile, flammable liquid made from crude oil or petroleum, used primarily as fuel in internal combustion engines.
Branched Alkane
Hydrocarbons featuring a tree-like structure, where carbon chains deviate from a straight chain due to side chains.
London Force
Weak, temporary van der Waals forces arising from the instantaneous polarization of molecules due to the movement of electrons.
Boiling Point
The temperature at which a liquid changes to a gas at a given pressure, characteristic for each specific substance.
Q17: Which of the following compounds might be
Q22: If 11.7 g of naphthalene,C<sub>10</sub>H<sub>8</sub>,is dissolved in
Q27: Given the equilibrium constants for the equilibria,
Q37: Which of the following is not an
Q41: Calculate the value of the reaction quotient,Q,for
Q44: The reaction A → B follows first-order
Q44: Arrange HF,HCl,and HBr in increasing order of
Q44: What is the freezing point of a
Q53: What mass of Zn(NO<sub>3</sub>)<sub>2</sub> must be diluted
Q62: For the equilibrium N<sub>2</sub>O<sub>4</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="For