Given the following equilibrium constants, Ka (HSO4-) = 1.2 × 10-2
Kb (CH3CO2-) = 5.6 × 10-10
Kw = 1.00 × 10-14
determine the equilibrium constant for the reaction below at 25 °C.
HSO4-(aq) + CH3CO2-(aq) 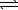 SO42-(aq) + CH3CO2H(aq)
SO42-(aq) + CH3CO2H(aq)
Definitions:
Sequential Integers
A series of integers (whole numbers) arranged in an ordered sequence where each number differs from the previous one by a constant step, typically one.
Number
An object in mathematics that is employed for counting, measuring, and labeling.
SeqNumber
Typically refers to a sequential number, an identifier used to denote the position of an item within a series or order of items.
Q22: Calculate the standard entropy change for the
Q25: Real gases are those that<br>A) only behave
Q39: Aluminum(III)ion (Al<sup>3+</sup>)is reduced to solid aluminum at
Q43: What is the coordination number of the
Q57: Which of the following species are likely
Q58: If the reaction quotient,Q,is greater than K
Q62: Which of the following sets of elements
Q69: The pressure exerted by gas molecules inside
Q69: What partial pressure of oxygen gas is
Q73: If 25 mL of 0.750 M HCl