The complete reaction of an acid and base is as follows. HNO3(aq) + LiOH(aq) → H2O( 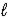 ) + LiNO3(aq)
) + LiNO3(aq)
What is the equilibrium constant for the net ionic reaction at 25 °C?
Definitions:
Registration Statement
A legal document filed with securities regulatory authorities disclosing important details about a company’s operations, securities, and management, primarily for the public and investors.
Management Buyout
An acquisition where a company's existing managers acquire a large part or all of the company from the current owners.
Corporate Stock
Equity securities issued by corporations, representing ownership in the company and rights to its profits.
Public
The general population or community at large, often referenced in the context of public services, spaces, or interests.
Q8: Which of the following water pollutants was
Q9: Calculate the value of the equilibrium constant
Q10: The value of E°<sub>cell</sub> is <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg"
Q13: A flask containing helium gas is released
Q13: Cesium crystallizes in the body-centered cubic system.If
Q15: What mass of an aqueous 18.8% glucose
Q35: Iron(II)sulfide has a primitive cubic unit cell
Q36: Which two of the following materials are
Q43: What is the osmotic pressure of an
Q45: A solution has a hydroxide ion concentration