Given the following acid dissociation constants: Ka (H3PO4) = 7.5 × 10-3
Ka (NH4+) = 5.6 × 10-10
Determine the equilibrium constant for the reaction below at 25 °C.
H3PO4(aq) + NH3(aq) 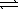 NH4+(aq) + H2PO4−(aq)
NH4+(aq) + H2PO4−(aq)
Definitions:
Account
An individual record within a financial system or ledger that tracks the financial transactions of a specific asset, liability, revenue, expense, or equity component.
Normal Balance
The side (debit or credit) on which increases to the account are recorded, consistent with the accounting equation.
Financial Statement
Reports that provide information about a company's financial condition, performance, and cash flows, including the balance sheet, income statement, and statement of cash flows.
Account
An account in accounting is a record within the general ledger that is used to collect and store debit and credit amounts.
Q5: Aqueous colloidal solutions can be classified as
Q10: What is the equilibrium constant for the
Q23: The mechanism of the chlorine atom catalyzed
Q34: What volume of a 0.758 M solution
Q34: What is the distance,in atomic radii,along any
Q42: Calculate the mass of coal that produces
Q65: If an electric current is passed through
Q70: The hydroxide ion concentration of a saturated
Q71: Calculate the pH of a 1.7 M
Q72: Which of the following statements is true