Given the following equilibrium constants, Ka (HSO4-) = 1.2 × 10-2
Kb (CH3CO2-) = 5.6 × 10-10
Kw = 1.00 × 10-14
determine the equilibrium constant for the reaction below at 25 °C.
HSO4-(aq) + CH3CO2-(aq) 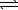 SO42-(aq) + CH3CO2H(aq)
SO42-(aq) + CH3CO2H(aq)
Definitions:
Interest Revenue
Income earned from investments, loans, or other interest-bearing financial assets.
Carrying Amount
The balance of the bonds payable account (face amount of the bonds) less any unamortized discount or plus any unamortized premium.
Gain
A financial increase resulting from a transaction that exceeds the costs or purchase prices of the assets sold, not related to the primary operations.
Long-Term
Refers to holding or affecting a period extending over a long duration, typically beyond one year.
Q29: A certain weak base B has a
Q37: What mass of solid NaCH<sub>3</sub>CO<sub>2</sub> (molar mass
Q40: Which of the following ions has the
Q47: Which of the following molecules has the
Q48: In the United States,most sulfur is obtained
Q57: For the reaction Br<sub>2</sub>(l)→ 2Br(g),_.<br>A) ΔH is
Q59: Batteries used in watches contain mercury(II)oxide.As the
Q62: For the following gas-aqueous liquid equilibrium for
Q63: What is the half-life of the first-order
Q70: The hydroxide ion concentration of a saturated