The complete reaction of an acid and base is as follows. HNO3(aq) + LiOH(aq) → H2O( 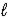 ) + LiNO3(aq)
) + LiNO3(aq)
What is the equilibrium constant for the net ionic reaction at 25 °C?
Definitions:
Every Year
An annually recurring period or event, indicating something that happens once every calendar year.
Absolute Confidentiality
The strict obligation to not disclose information shared in a private setting to any third parties without clear consent.
Legal Proceedings
Activities that seek to invoke or are in accordance with the law, including trials, hearings, and other formalities leading to the enforcement or recognition of legal rights.
Conditional Confidentiality
An agreement in counseling or research settings where the expectation of privacy is subject to certain exceptions, usually involving the safety of the individual or others.
Q2: Which one of the following sets of
Q12: Point D on the phase diagram is
Q21: Consider the exothermic combustion of coal.Which of
Q22: How many unpaired electrons are found in
Q30: Which of the following pairs of coordination
Q31: When the pressure of an equilibrium mixture
Q39: If Δ<sub>r</sub>G° > 0 for a reaction
Q44: The Ostwald process is an industrial method
Q60: For a certain reversible process,q = 88.06
Q76: Which of the following balanced chemical equations