Exactly 1.0 mol N2O4 is placed in an empty 1.0-L container and allowed to reach equilibrium described by the equation N2O4(g) 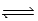 2NO2(g) . If at equilibrium the N2O4 is 27.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?
2NO2(g) . If at equilibrium the N2O4 is 27.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?
Definitions:
Owned Content
Media and content that a company creates and controls, such as websites, blogs, and social media profiles.
Volume Of Mentions
A metric that quantifies how often a brand, product, or topic is mentioned across various media platforms within a specific timeframe.
Informed Consent
A process by which individuals are fully informed about the procedures and risks involved in a treatment or experiment before agreeing to participate.
Spam
Unwanted or unsolicited digital messages, often sent in bulk for promotional purposes.
Q21: Write a balanced half-reaction for the reduction
Q28: Molecules must overcome a barrier called the
Q32: The vapor pressure of nitric acid is
Q49: One commercial method of producing Cl<sub>2</sub> is
Q51: Write a balanced chemical equation for the
Q51: For a reaction,Δ<sub>r</sub>H° = -208.8 kJ and
Q64: For a certain overall second-order reaction with
Q64: At 25 °C,0.138 mg AgBr dissolves in
Q70: What is the vapor pressure at 20°C
Q78: Which of the following is true for