At 25 °C,0.138 mg AgBr dissolves in 10.0 L of water.What is the equilibrium constant for the reaction below? AgBr(s) 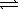 Ag+(aq) + Br-(aq)
Ag+(aq) + Br-(aq)
Definitions:
Ethic Reciprocity
the principle of treating others as one would wish to be treated oneself.
Visibility
The awareness of others regarding your presence in an organization.
Underlying Norms
Fundamental, often unspoken rules that govern behavior within a group or society.
Political Situation
A scenario characterized by the distribution of power and authority among individuals or groups and the dynamics of political actions and decisions.
Q2: Which of the following units are consistent
Q8: Termolecular elementary steps are rare.Why?
Q31: Henry's Law constant is 0.0013 mol/kg⋅bar and
Q42: On the phase diagram below,which point corresponds
Q45: For which of the following hypothetical rate
Q48: In the United States,most sulfur is obtained
Q62: What is the hybridization of each carbon
Q63: For a chemical system,Δ<sub>r</sub>G° and Δ<sub>r</sub>G are
Q68: Calculate <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="Calculate for
Q72: Which combination of atoms is most likely