In an experiment,0.46 mol H2 and 0.46 mol I2 are mixed in a 1.00-L container,and the reaction forms HI.If Kc = 49.for this reaction,what is the equilibrium concentration of HI? I2(g) + H2(g) 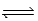 2HI(g)
2HI(g)
Definitions:
Processing Department
A specific unit within a manufacturing facility where a particular process or sequence of operations is carried out.
Weighted-Average Method
An inventory costing method that calculates the cost of goods sold and ending inventory based on an average cost of all goods available for sale.
Total Cost Transferred
The total amount of costs moved from one stage of production to the next, or from one department to another.
Processing Department
A division within a factory where a specific stage of production is completed, contributing to the transformation of raw materials into finished products.
Q5: What is the hybridization of oxygen atom
Q6: As pure molecular solids,which of the following
Q18: For a particular liquid,raising its temperature from
Q21: Ethanol has an enthalpy of vaporization of
Q29: Which of the following is not a
Q43: Calculate E°<sub>cell</sub> for the cell for the
Q47: A physics experiment is conducted at a
Q51: How many mechanistic steps are depicted by
Q60: Which of the following substances is never
Q69: The average rate of disappearance of ozone