At 25 °C,the decomposition of dinitrogen tetraoxide N2O4(g) 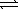 2 NO2(g)
2 NO2(g)
Has an equilibrium constant (Kp) of 0.144.At equilibrium,the total pressure of the system is 0.0758 atm.What is the partial pressure of each gas?
Definitions:
Framing Effects
The influence on an individual's decision-making caused by the way information is presented, rather than just the information itself.
Status Quo
The existing state of affairs; in prospect theory, the current situation from which gains and losses are calculated.
Utility
A measure of satisfaction or value that consumers receive from consuming goods and services.
Endowment Effect
The tendency people have to place higher valuations on items they possess (are endowed with) than on identical items that they do not possess; perhaps caused by loss aversion.
Q8: A 26.4-L sample of nitrogen at 7.50
Q10: Using the given data,determine ΔrG° at 298
Q12: A molecule will always be polar if
Q19: Write a balanced chemical equation which corresponds
Q44: What is the freezing point of a
Q53: Which of the following molecules has the
Q56: The pH of aqueous 0.10 M pyridine
Q68: When a water molecule forms a hydrogen
Q73: At 25 °C,all of the following ions
Q78: All of the following compounds are acids