The standard enthalpy of formation of ammonia is -46.1 kJ/mol.
1/2 N2(g)+ 3/2 H2(g) 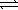 NH3(g)
NH3(g)
Commercially,the reaction is carried out at high temperatures.Using your knowledge of kinetics and equilibrium,explain an advantage and a disadvantage of synthesizing ammonia at high temperatures.
Definitions:
Movable Collateral
Assets that can be relocated and are used as security for a loan without transferring ownership to the lender.
Secured Party's Remedies
Legal actions or measures available to a secured party to enforce rights against a debtor in case of default.
Debtor Defaults
A situation where a debtor is unable to meet the legal obligation of debt repayment.
After-Acquired Property
Property acquired by a debtor after the execution of a security agreement, which may still be subject to claims by secured creditors under certain agreements.
Q5: For a certain reaction of the general
Q6: Which of the following methods is used
Q23: What is the formal charge on each
Q34: Which of the following is the bond
Q38: The pressure of O<sub>2</sub> in a 15.0
Q40: Consider the following equilibria. PbBr<sub>2</sub>(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg"
Q43: An impure sample of sodium carbonate,Na<sub>2</sub>CO<sub>3</sub>,is titrated
Q64: Calculate the equilibrium constant for the reaction
Q75: A vessel with a volume of 17.3
Q76: What is the K<sub>c</sub> expression for the