At a high temperature,equal concentrations of 0.160 mol/L of H2(g) and I2(g) are initially present in a flask.The H2 and I2 react according to the balanced equation below. H2(g) + I2(g) 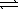 2 HI(g)
2 HI(g)
When equilibrium is reached,the concentration of H2(g) has decreased to 0.036 mol/L.What is the equilibrium constant,Kc,for the reaction?
Definitions:
Stylistic Approach
The unique manner or technique an artist, writer, filmmaker, or musician uses to express their vision or tell a story, often characterized by distinct features or elements.
Federal Government
The national government of a federation, which holds authority over matters that affect the entire country, functioning as the central governing body.
Hollywood Studios
Major film production and distribution companies based in Hollywood, California, known for producing a significant portion of the American film industry's output.
WWII
A global military conflict that lasted from 1939 to 1945, involving most of the world's nations and resulting in significant loss of life and widespread destruction.
Q7: What is the pOH of 0.074 M
Q17: If a catalyst is present in a
Q18: In metals,there are not enough electrons to
Q19: What volume of water must be added
Q21: Which diatomic molecule or ion has valence
Q31: Sulfur dioxide has a vapor pressure of
Q40: The change in entropy for any process
Q43: At 800 K,the equilibrium constant,K<sub>p</sub>,for the following
Q51: In HF<sub>2</sub>− the hydrogen is shared between
Q74: Write a balanced net ionic equation for