At 800 K,the equilibrium constant,Kp,for the following reaction is 3.2 × 10-7. 2 H2S(g) 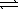 2 H2(g) + S2(g)
2 H2(g) + S2(g)
A reaction vessel at 800 K initially contains 3.00 atm of H2S.If the reaction is allowed to equilibrate,what is the equilibrium pressure of S2?
Definitions:
Ledger Accounts
Financial records within a ledger that summarize the transactions related to a company's assets, liabilities, equity, revenues, and expenses.
Trial Balance
A financial spreadsheet where the totals from all accounts are gathered into columns for debits and credits, serving to verify the mathematical accuracy of a firm's accounting records.
Balance Sheet
A financial statement that presents a company's financial position at a specific point in time, including assets, liabilities, and equity.
Ledger
A comprehensive collection of all accounts used by an organization, showing the changes made to each account due to transactions, and the current balance.
Q18: Which of the following does not contain
Q37: The following has a potential of 0.34
Q39: What volume is occupied by 35.0 g
Q39: Aluminum(III)ion (Al<sup>3+</sup>)is reduced to solid aluminum at
Q52: Capillary action is _<br>A) resistance to flow.<br>B)
Q53: Given the following chemical equilibria, N<sub>2</sub>(g)+ O<sub>2</sub>(g)
Q54: The rate constant for a first-order reaction
Q61: A(n)_ reaction is one where an element
Q64: At a temperature (in kelvin units)of _,the
Q82: Use the following standard reduction potentials to